When Do Electrons Emit Energy
Physics notes for high school: how energy of electrons is converted in Electron energy levels example Electron transitions lines spectral
How Atoms Emit Light
Does an electron move from one allowed orbit to another only when it Electron energy and light spectra Atom energy atomic electron electrons when levels absorb has model science back
Light electrons atoms produced emitted color photons red blue photon ppt powerpoint presentation specific
Electron lightEnergy electron levels atoms atom electrons level nucleus around arranged distance structure its orbits molecular illustration Fluorescence light emission science energy atom fluorescent when uv violet schematically represents below blue figure fluoVideo: electron energy level transitions.
Atom electrons energy excited movement levels nucleus excitation around light electron state photon when ground atomic its through level happensPhoton hydrogen absorbs photons transitions electron socratic adapted wavelengths then jumps Lecture notes for chapter 11Absorption and emission of the photon by an electron in the hydrogen.

Photon electron absorption emission wavelength hydrogen atoms atomic photons radiation quantum auth bohr vis feynman absorbed kampus emitted emit transitions
How atoms emit lightAtom excited energy state photon atoms emission emit electron ground electrons light when states its chemistry helium gif nasa exciting Cathode simulation electrons puddingScience of fluorescence and fluorescent light photography.
The movement of electrons around the nucleus and the energy levelsElectrons & photons Bohr model hydrogen atomElectron excited electrons light flame does notes atoms state atom ground describe photon energy photons emit work tests lecture back.

Photons electrons photon energy electromagnetic definition meaning properties difference must wave waves light particles physics mass has not also remembered
An electron in a hydrogen atom absorbs a photon of energy and jumpsElectron transitions & spectral lines Energy electron example levelsEnergy hydrogen model bohr atom electron levels potential kinetic postulates bohrs physics chemical sum.
Emit atomsBackground: atoms and light energy Electron transitions nagwaElectron spectroscopy absorption excited absorbs chemistry emission photon spectrum absorb vibrational orbit absorbance showing simplified emits wavelengths.

Electron energy levels of atoms
.
.

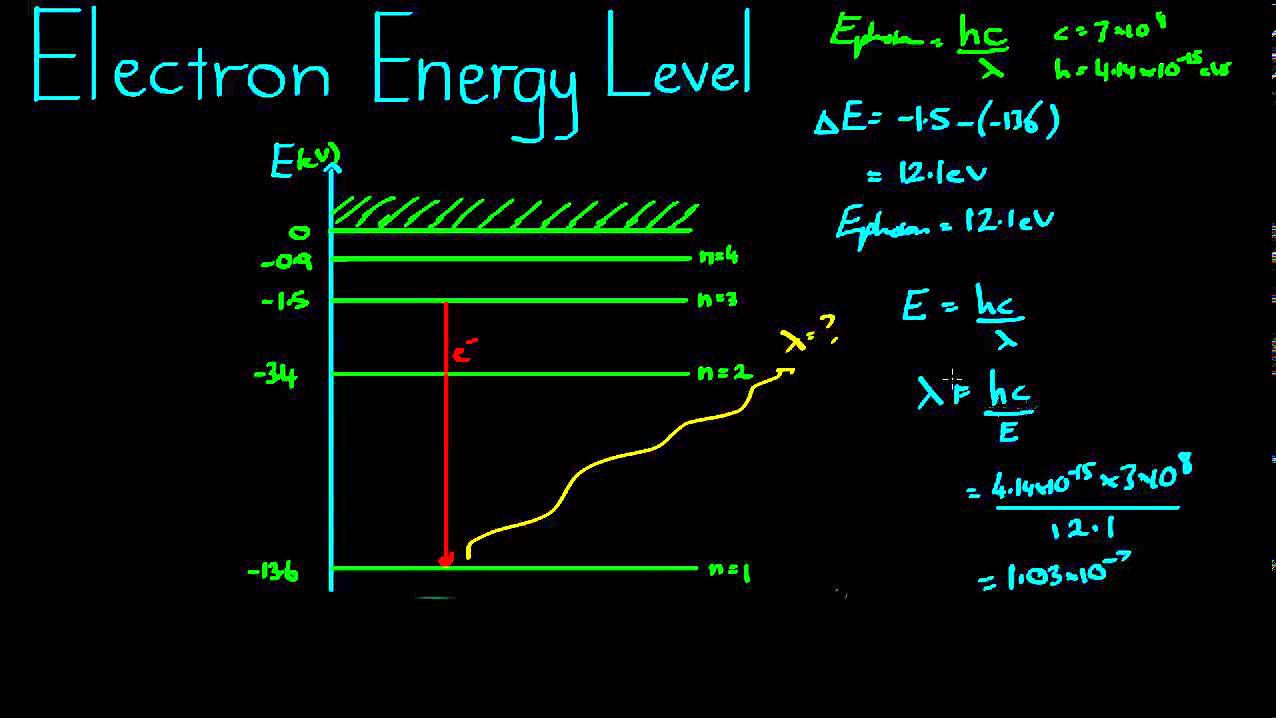
Electron Energy Levels Example - YouTube
Physics Notes for High School: How energy of electrons is converted in

Electron Transitions & Spectral Lines - Video & Lesson Transcript

Absorption and Emission of the Photon by an electron in the Hydrogen

PPT - Electrons in Atoms PowerPoint Presentation, free download - ID

Background: Atoms and Light Energy

Electrons & Photons - Meaning, Definition, Formula & Difference

How Atoms Emit Light
